Can the shape of a crystal really change how well it performs in clean energy technology? A new study says yes—decisively.
Researchers from National Taiwan University, National Tsing Hua University, and National Yang Ming Chiao Tung University have discovered that the performance of a widely studied catalyst, cuprous oxide (Cu2O), in oxygen reduction reactions (ORR) depends heavily on which crystal face is exposed.
The paper is published in the Journal of Materials Chemistry A.
Oxygen reduction is a central reaction in fuel cells, which are devices that convert chemical energy into electricity. Platinum is commonly used in this role but is expensive and limited in supply. Cu2O, a more affordable alternative, has now shown surprising potential—if used with the right shape.
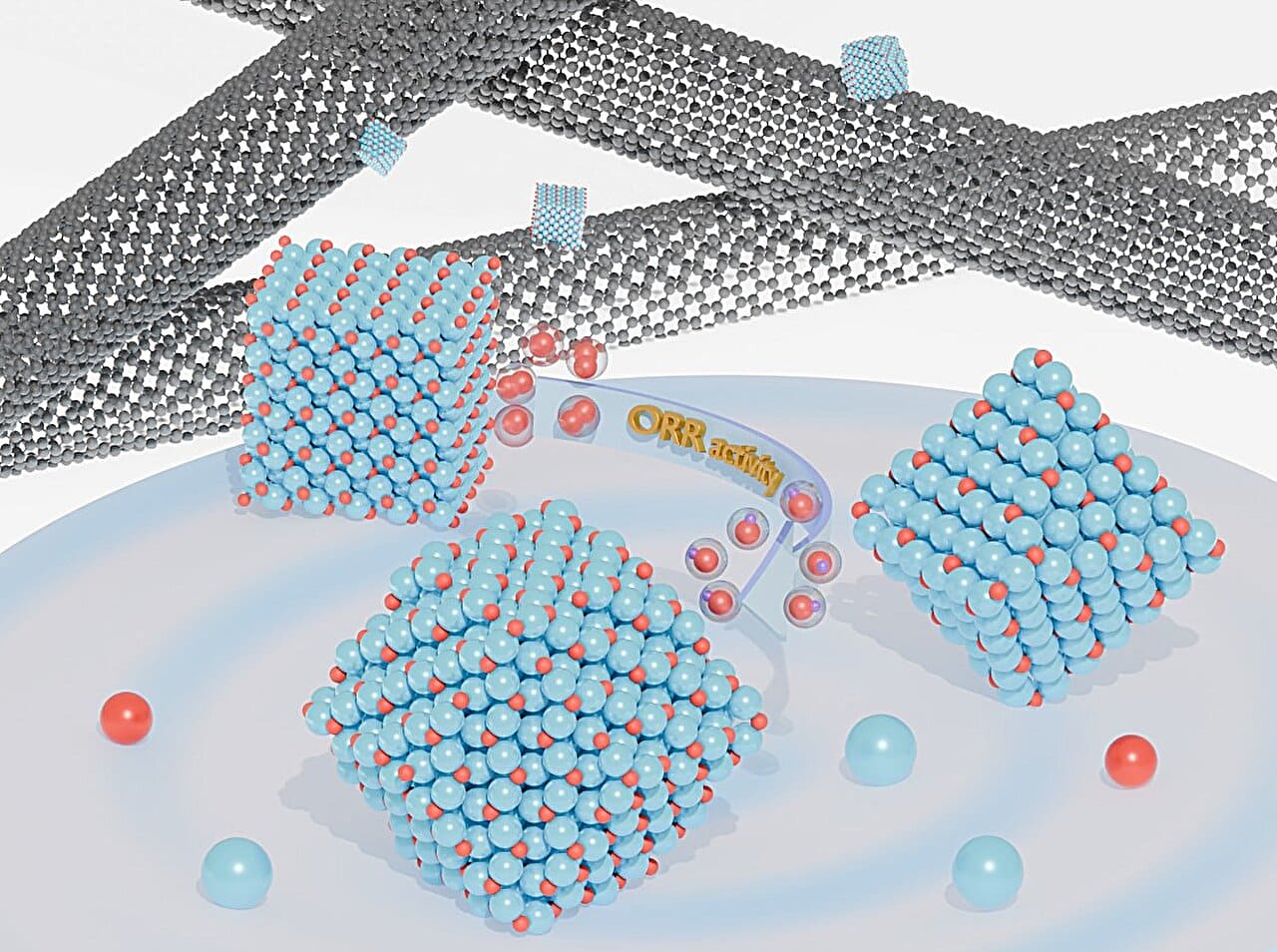
The team synthesized Cu2O crystals into three distinct shapes: cubes, octahedra, and rhombic dodecahedra. These shapes expose different crystal facets—{100}, {111}, and {110}, respectively—and were each blended with carbon nanotubes to enhance conductivity.
The researchers found that the rhombic dodecahedron version, exposing the {110} surface, delivered the strongest catalytic activity for ORR, while the cube was most stable over time.
By combining advanced quantum simulations with lab experiments, the team found that oxygen molecules behave differently depending on which crystal surface they land on.
The {110} surface showed the weakest grip on oxygen, which helps the reaction proceed more smoothly. This matched their density functional theory (DFT) predictions and was clearly shown in free energy diagrams and 2D volcano plots that link binding strength to catalytic performance.
However, better performance sometimes comes at a cost. The rhombic dodecahedron crystals were found to degrade faster during operation, possibly due to self-oxidation. In contrast, the cube-shaped crystals—though less reactive—were more robust over time.
This research not only helps explain why different facets perform differently but also opens doors to designing next-generation, low-cost catalysts by simply controlling crystal shapes.
"By fine-tuning the geometry of crystal surfaces, we can tailor their reactivity and stability, which is crucial for advancing sustainable energy technologies," said Prof. Jyh-Pin Chou of National Taiwan University.
More information: Chih-Chun Chang et al, Specific Cu2O surfaces for electrocatalytic oxygen reduction reaction, Journal of Materials Chemistry A (2025). DOI: 10.1039/D4TA08855G
Journal information: Journal of Materials Chemistry A
Provided by National Taiwan University