A research team from National Taiwan University, led by Prof. Chih-Jung Chen has developed an innovative electrochemical platform capable of efficiently converting biomass into high-value chemicals while simultaneously generating hydrogen fuel—without the use of conventional electrolytes or ion-exchange membranes.
This advancement offers a promising solution to longstanding challenges in sustainable chemical production and clean energy technologies.
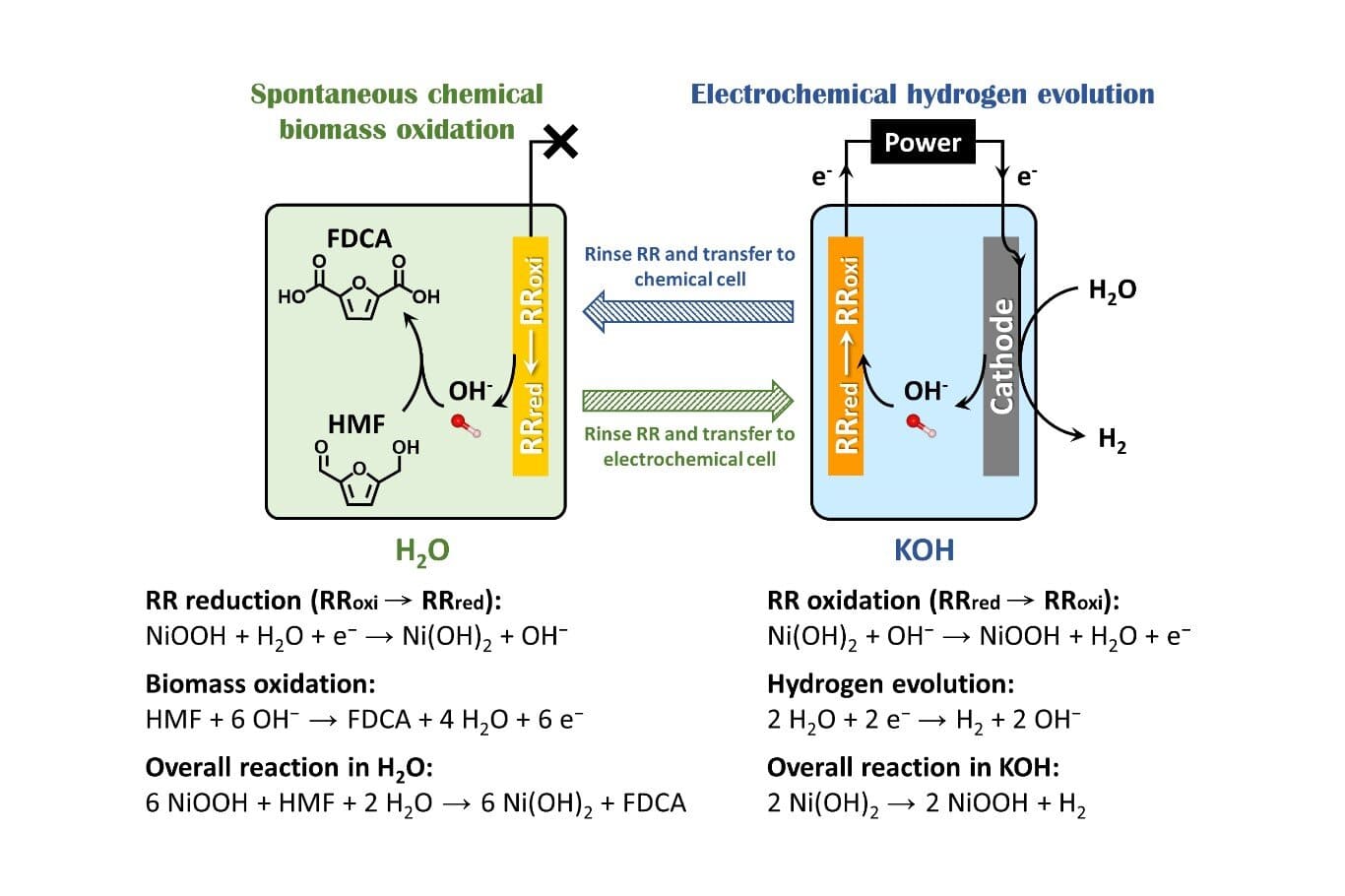
In their study published in the Chemical Engineering Journal, the team introduced a "redox reservoir" (RR) system that decouples the oxidation of 5-hydroxymethylfurfural (HMF)—a key biomass-derived compound—from the hydrogen evolution reaction (HER).
By separating these two half-reactions both spatially and temporally, the system minimizes undesirable side reactions and allows greater control over each process.
Traditionally, HMF oxidation is carried out in strongly alkaline electrolytes to enhance reaction rates. However, such environments often trigger unwanted side reactions like Cannizzaro disproportionation and humin formation, reducing product yield and carbon efficiency. Moreover, the cathodic HER process can further destabilize HMF molecules, leading to significant carbon loss.
To address these limitations, the researchers designed a reusable RR electrode composed of nickel oxyhydroxide (NiOOH), which serves as a solid-state oxidant.
In pure water, the RR chemically oxidizes HMF without the need for electrolyte salts or external voltage, undergoing conversion to nickel hydroxide [Ni(OH)2]. This reduced form of the RR can then be electrochemically regenerated during HER, thereby completing the redox cycle.
"The concept is similar to pumped hydro storage, but implemented at the microscale," explained lead author Shih-Wei Lin. "Energy is stored electrochemically in the RR electrode during HER and later released chemically to drive biomass oxidation—efficiently and cleanly."
The platform demonstrated remarkable performance, achieving a 97.4% yield of 2,5-furandicarboxylic acid (FDCA)—a key monomer for bioplastics—from HMF concentrations as high as 300 mM, approaching industrially relevant levels.
During the HER step, the regeneration of NiOOH maintained a Faradaic efficiency of 96.0%, while the overall process achieved a high voltage efficiency of 94.8%.
"Our findings open new avenues for green chemical synthesis," said Prof. Chih-Jung Chen. "The system is also capable of oxidizing other organic molecules containing aldehyde or alcohol groups, such as furfural, underscoring its versatility."
By eliminating supporting electrolytes and membrane components, the approach reduces energy demands, lowers material costs, and simplifies downstream purification. It also minimizes carbon losses and enhances product purity, offering a scalable and sustainable pathway for producing bio-based chemicals and clean hydrogen.
This breakthrough represents a critical step toward decarbonizing the chemical industry and leveraging renewable electricity for environmentally responsible chemical transformations.
More information: Shih-Wei Lin et al, Stepwise chemical-electrochemical cycles for decoupling modular biomass oxidation and hydrogen evolution, Chemical Engineering Journal (2024). DOI: 10.1016/j.cej.2024.158764
Journal information: Chemical Engineering Journal
Provided by National Taiwan University