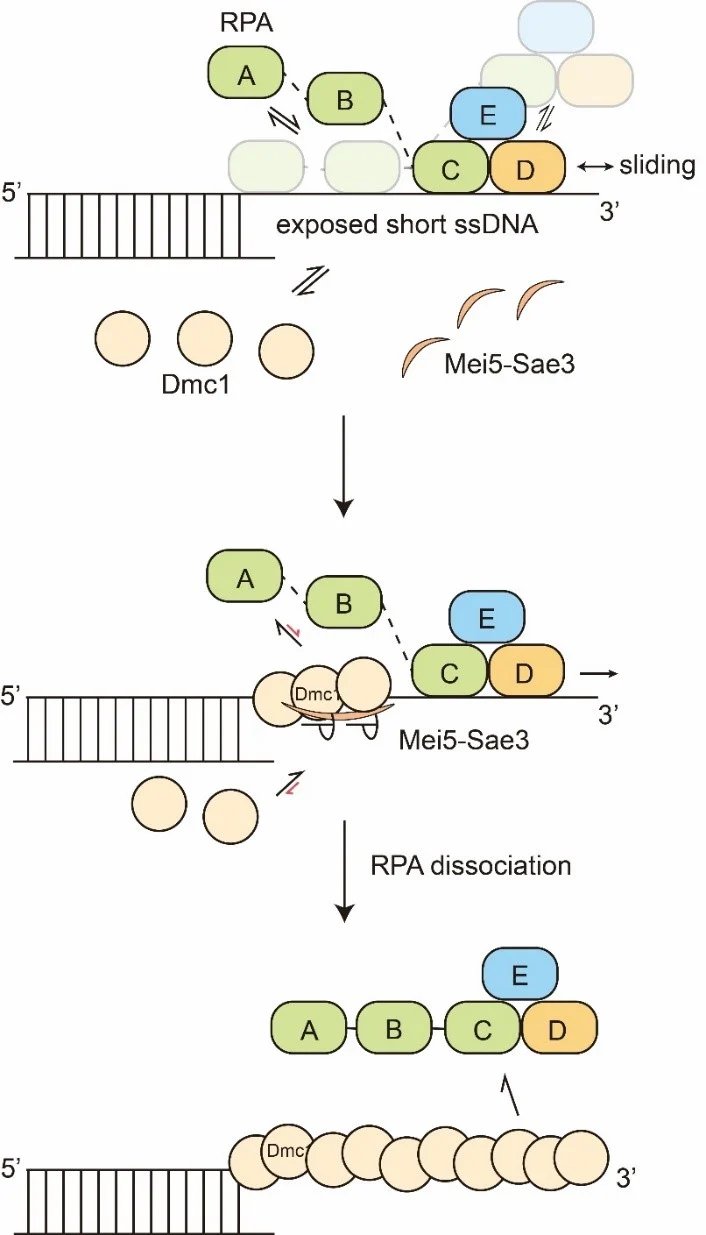
How multiple DNA binding proteins compete for the DNA substrate leads to different biochemical outcomes. Using single-molecule experiments, this work demonstrates that the regulatory protein Mei5-Sae3 complex stabilizes Dmc1 recombinases on RPA-coated DNA, leading to efficient RPA displacement and Dmc1 assembly, which in turn stimulates recombination progression.
Meiotic recombination generates genetic diversity and promotes proper chromosomal segregation of parental chromosomes. This process requires a set of recombinases polymerized on single-stranded (ss)DNAs called the nucleoprotein filament to undergo homology search and strand exchange between homologous DNAs. In Saccharomyces cerevisiae meiosis, programmed DNA double-strand breaks (DSBs) are formed by Spo11 to generate 3’-ssDNA tails. Once formed, ssDNA overhangs are rapidly bound by the abundant high-affinity ssDNA-binding protein, Replication protein A (RPA), to protect these ssDNAs from nucleolytic degradations or formation of the higher-order DNA structures. RPA-coated ssDNA substrates are distinct from bare ssDNA substrates due to RPA's high affinity for ssDNA; therefore, recombination mediator Mei5-Sae3 protein complex is required for the binding of recombinases onto RPA-coated ssDNA. However, the mechanistic role of Mei5-Sae3 in mediating Dmc1 activity remains unclear.
To investigate how Mei5-Sae3 stimulates Dmc1 to displace RPA and form nucleoprotein filaments, the research team consists of NTU chemistry, NTU IBS, and Osaka University utilizes Biochemical protein purification techniques, and single-molecule FRET and Colocalization Single-Molecule Spectroscopy (CoSMoS) techniques to real-time capture the binding of Dmc1 and dissociation of RPA on individual DNA with exceptional time resolution. Unlike traditional biological approaches, which mostly look at the final equilibrium products of the ensemble reactions, single-molecule methods could elucidate the contributions of individual biochemical steps from individual molecules, uncovering transient intermediate states that could give insights into how the reaction progresses.
The result showed that Mei5-Sae3 stabilized Dmc1 nucleating clusters with 2-3 molecules on naked DNA by preferentially reducing Dmc1 dissociation rates. Mei5-Sae3 also stimulated Dmc1 assembly on RPA-coated DNA. Using GFP-labelled RPA, the co-existence of an intermediate with Dmc1 and RPA on ssDNA was observed before RPA dissociation. Moreover, the displacement efficiency of RPA depended on Dmc1 concentration, and its dependence was positively correlated to the stability of Dmc1 clusters on short ssDNA. These findings suggest a molecular model that Mei5-Sae3 mediates Dmc1 binding on RPA-coated ssDNA by stabilizing Dmc1 nucleating clusters, thereby influencing RPA dynamics on DNA to promote RPA dissociation. This research contributes to the first ever reported detailed molecular model for this unique mediator protein Mei5-Sae3, elucidating how a mediator-recombinase interaction can stimulate recombinase assembly on RPA-coated ssDNA.
The co-first authors of this research include Chin-Dian Wei, an undergraduate researcher (NTU Chemistry), and Dr. Hao-Yen Chang, a postdoctoral fellow (NTU IBS and Chemistry). Other contributing authors are Chia-Hua Lu, Chih-Chun Chang, and the Japanese team Asako Furukohri and Stephen Mwaniki from Professor Akira Shinohara’s lab at Osaka University. This research is supported by NSTC, NTU, and Osaka University.
Link to the paper: https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkae780/7757939