Organic light-emitting diode (OLED) displays have been applied in smartphones, computers, and televisions. However, the current use of iridium-containing light-emitting materials has led to high production costs for OLED displays. Therefore, it is imperative to develop low-cost, high-efficiency pure organic light-emitting materials. Among them, organic materials capable of exhibiting thermally activated delayed fluorescence (TADF) are highly anticipated. This fluorescence characteristic can significantly improve the efficiency of converting electrical energy into light energy, leading to high-efficiency OLED devices with great commercial potential. In addition to complex synthesis pathways for organic molecules with this property, it is also possible to achieve TADF by physically mixing mixtures of molecules with electron-donating properties (donor, D) and molecules capable of accepting electrons (acceptor, A). Exciplexes formed by charge transfer from D to A under light excitation can also exhibit TADF. However, scientists have long been unable to determine the actual molecular structure of exciplexes, making it very difficult to study the relationship between exciplex structure and light-emitting properties, significantly limiting the research and development of exciplexes.
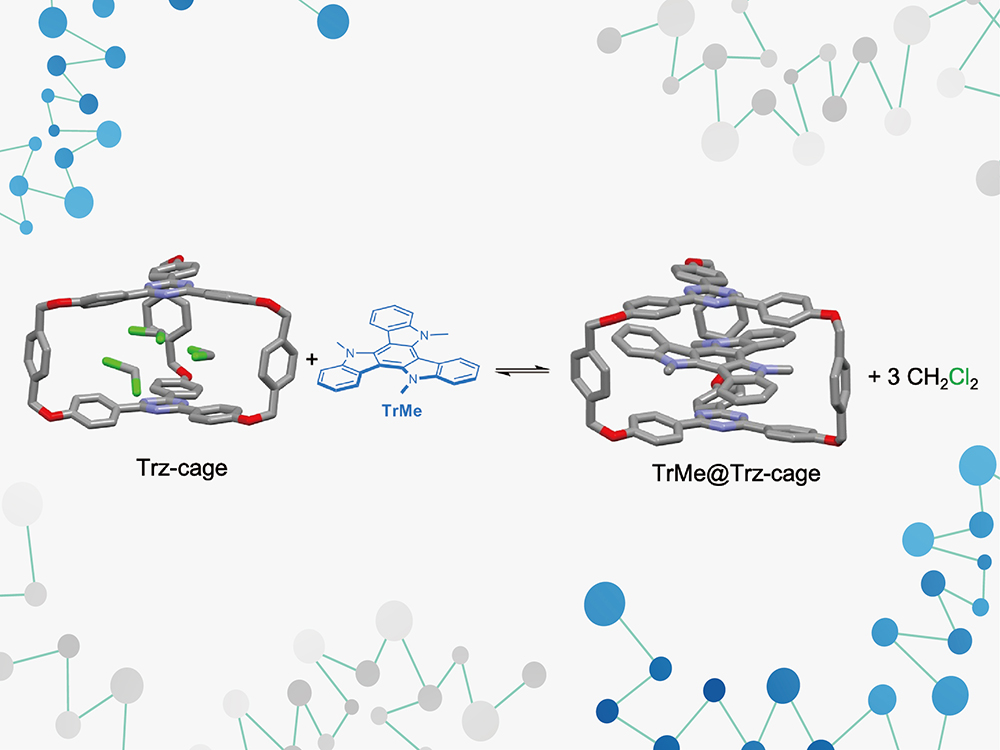
To address this issue, Professor Ken-Tsung Wong and the research team of Professor Pi-Tai Chou from the Department of Chemistry at National Taiwan University utilized electron-deficient molecules to design highly symmetrical cage-shaped molecules (Trz-cage) and synthesized them. The central space of this cage-shaped molecule can be used to capture molecules with electron-donating properties (TrMe) to form an inclusion supramolecular structure (TrMe@Trz-cage). Through the analysis of crystal structures, detailed arrangements and distances between the two molecules were obtained. This tightly embedded inclusion interaction enabled the study of the optical properties of D/A mixtures in both ground and excited states, and thermodynamic parameters for the formation of exciplexes in the ground state were successfully obtained.
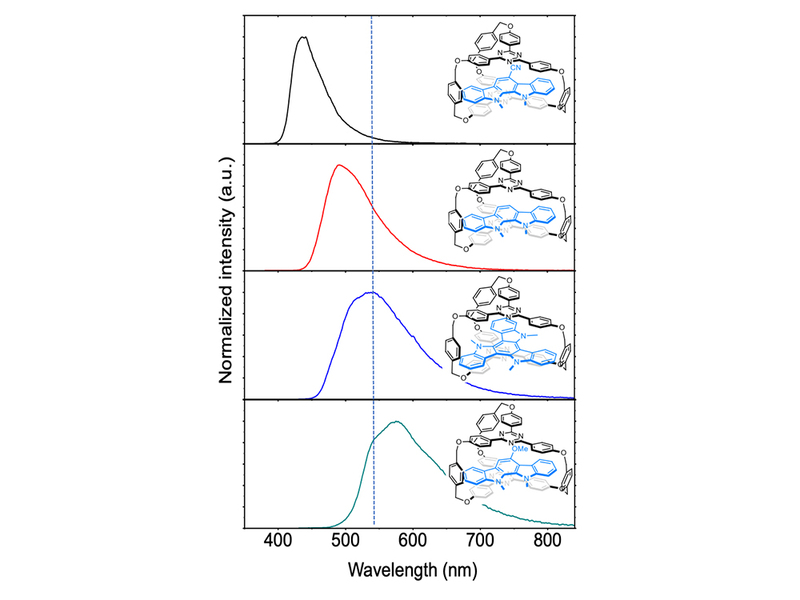
The research team further discovered that the enthalpy unfavorable to intermolecular interactions, but by pushing out three solvent molecules originally in the cage-shaped molecule with one D molecule, the reaction entropy increased, thereby driving the formation of inclusion complexes. This result illustrates that previous attempts to develop exciplexes using D/A mixtures failed to form a stable ground state under the dual unfavorable conditions of enthalpy and entropy, making it impossible to thoroughly study the structure and formation conditions of exciplexes. The research team can simply use different electron-deficient D molecules to control the emission color of the formed inclusion supramolecular structure, demonstrating the diversity of this new system and making it more accurate to explore the correlation between its light-induced optical properties and molecular structure.
This groundbreaking research breakthrough, breaking free from the shackles of previously unknown interactions between exciplex molecules, lays an important foundation for the future application of exciplexes in OLEDs, highlighting National Taiwan University's key role in the world stage of light-emitting material research and improving the brightness and cost of current OLEDs. The research results have been published in the flagship journal Nature Chemistry.
Link to the paper: https://www.nature.com/articles/s41557-023-01357-0
Source: https://www.ntu.edu.tw/spotlight/2024/2260_20240424.html