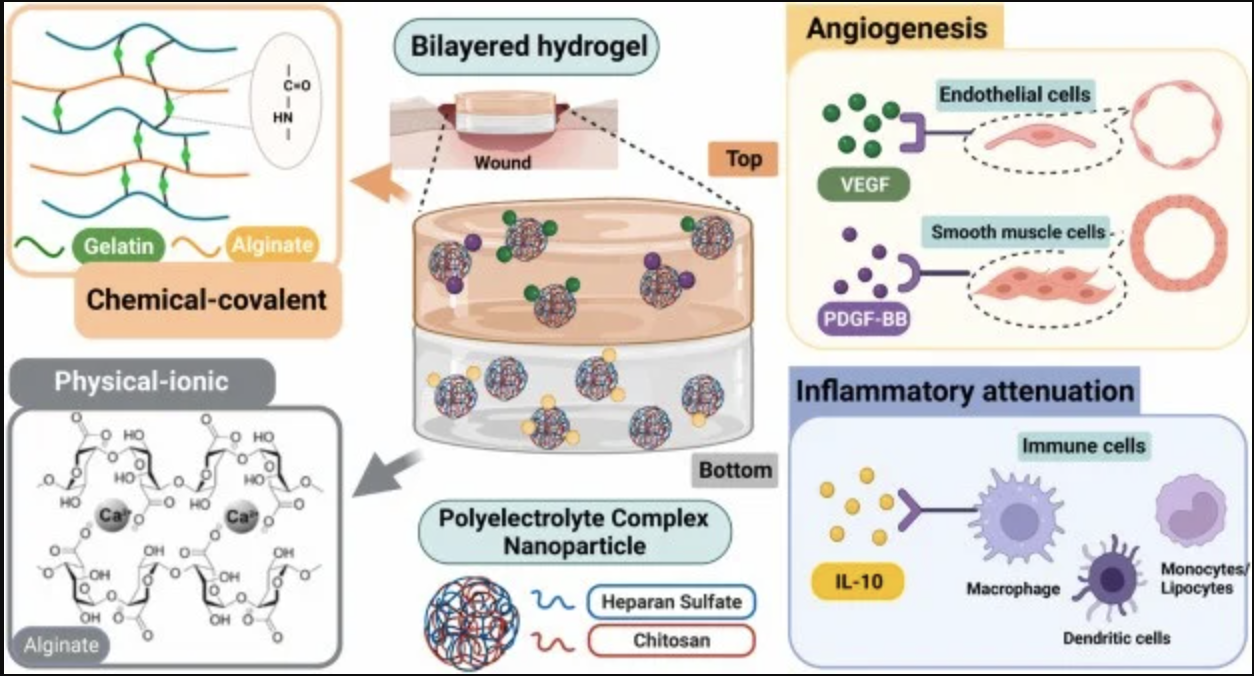
A bilayer alginate hydrogel system encapsulating polyelectrolyte complex nanoparticles (PCNs) loaded with anti-inflammatory cytokines and angiogenic growth factors, which reducing inflammation, stimulating angiogenesis, and accelerating wound closure in a diabetic murine model.
Chronic wounds, particularly in diabetic patients, present a significant medical challenge due to their persistent inflammation, impaired angiogenesis, and delayed healing processes. Recent advances in regenerative medicine have introduced a bilayer alginate hydrogel system that offers a promising solution for improving wound healing.
This innovative hydrogel encapsulates polyelectrolyte complex nanoparticles (PCNs) loaded with a combination of anti-inflammatory cytokines and angiogenic growth factors, creating an ideal microenvironment for tissue repair.
In a diabetic murine model, this hydrogel demonstrated remarkable therapeutic effects. The dual-layered design of the hydrogel not only supports the controlled release of the bioactive molecules but also enhances the healing process by targeting several key factors involved in wound recovery.
By reducing inflammation, the hydrogel prevents the chronic inflammatory state that often impairs tissue repair in diabetic wounds. It also polarizes macrophages toward a pro-regenerative phenotype, creating a microenvironment crucial for initiating tissue repair and resolving inflammation effectively.
Further studies revealed that the hydrogel facilitates keratinocyte migration, an essential step for the formation of new epithelial tissue and wound closure. Additionally, the angiogenic growth factors embedded within the PCNs stimulate the formation of new blood vessels, improving oxygen and nutrient supply to the wound site and promoting tissue regeneration.
Not only does this innovative hydrogel system reduce inflammation and enhance cellular migration, but it also accelerates the wound closure process, offering a potential therapeutic strategy for chronic wounds of diabetic patients.
The success of this approach in a murine model offers hope for future clinical applications in human wound care, potentially revolutionizing the treatment of difficult-to-heal diabetic ulcers and other chronic wounds.
The next step in this research will focus on refining the hydrogel’s components for enhanced stability and controlled release, as well as conducting clinical trials to assess its safety and efficacy in human patients. This work could pave the way for more effective wound healing therapies, drastically improving the quality of life for individuals suffering from chronic wounds.
Prof. Chen-Hsiang Kuan’s email address: chkuan0408@gmail.com