Researchers at National Taiwan University have developed a modular platform to reprogram tumor-derived extracellular vesicles (EVs), transforming them from oncogenic messengers into safe, customizable drug delivery vehicles through precise molecular editing.
In the bloodstream, microscopic particles called extracellular vesicles (EVs) constantly drift—tiny capsules released by our cells to carry messages, sometimes of healing, sometimes of harm. Among them, tumor-derived EVs are some of the most elusive: silent emissaries of cancer, cloaked in biological complexity and loaded with molecules that can drive disease progression.
But what if we could not only intercept these molecular messengers, but edit their contents and redirect their purpose?
A research team led by Dr. Chi-An Cheng at National Taiwan University's School of Pharmacy has just made that possible. In their new study in Advanced Functional Materials, the team unveils an integrated strategy to functionally reprogram tumor EVs—transforming them from high-risk, oncogenic carriers into precise, biocompatible drug delivery tools.
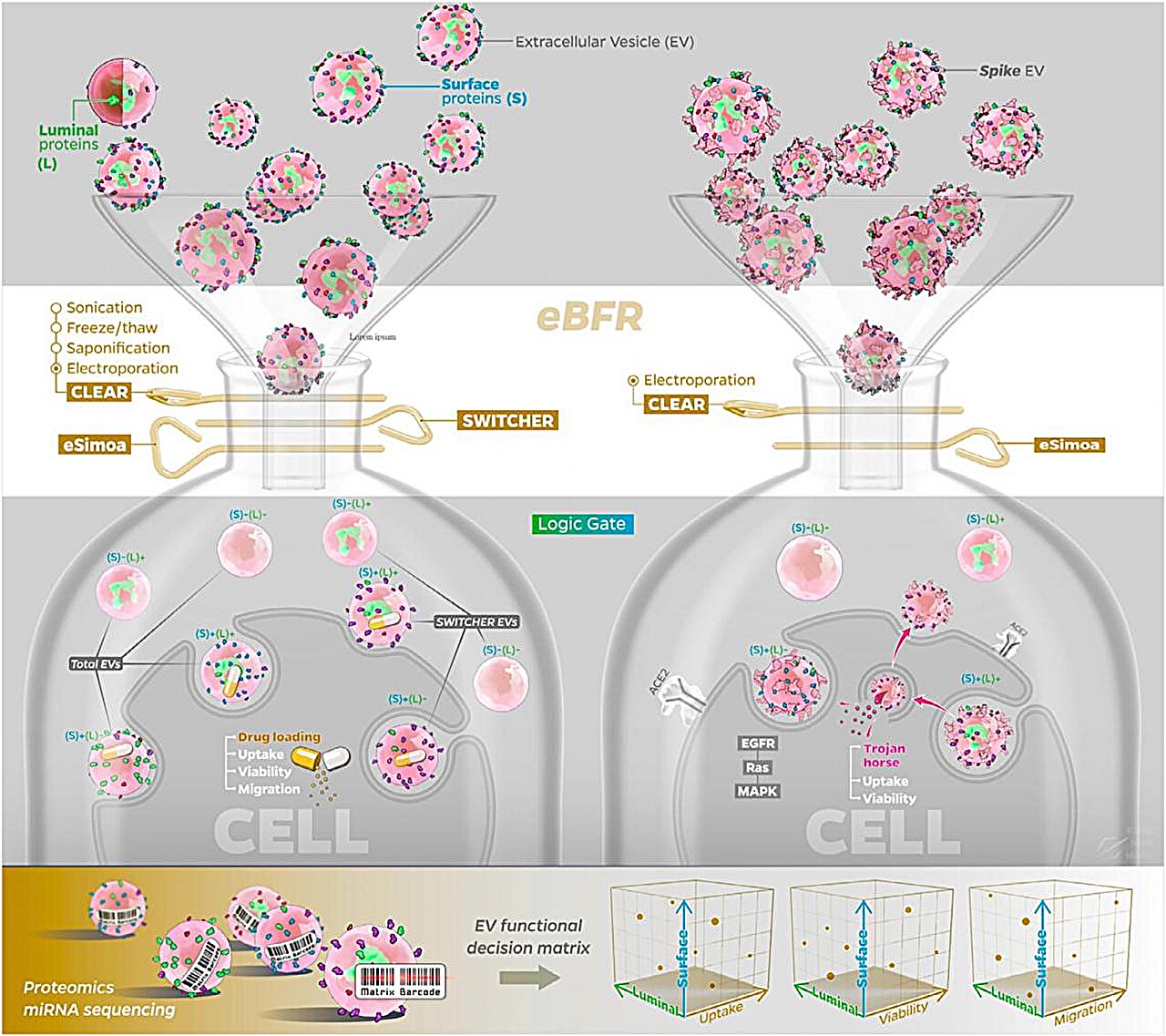
Their solution is the EV Bimodal Functional Regulator (eBFR) platform, which tackles a fundamental challenge in EV biology: their internal and external components can play vastly different roles, but are often analyzed as a whole. By separating and selectively modifying each part, Cheng's team is able to fine-tune EVs with unprecedented precision.
The platform combines: CLEAR, a method that removes dangerous internal cargo while preserving the vesicle's surface features; SWITCHER, a novel purification system that gently selects EVs bearing specific surface proteins; and eSimoa, a high-resolution protein profiling technology that measures EV components down to the single-molecule level.
Together, these tools allow researchers to map EV functions in three dimensions—surface vs. luminal, quantitative vs. spatial, biological vs. therapeutic—and, more importantly, to engineer EVs as programmable nanocarriers for cancer therapy.
In preclinical models, these reprogrammed EVs achieved higher drug payloads and better anti-tumor efficacies—an advance toward safer, more personalized treatments.
As Dr. Cheng notes, "We're not just studying EVs anymore—we're shaping them."
With this work, her team not only answers questions about EV biology, but also lays the groundwork for next-generation EV-based therapeutics that are both intelligent and adaptable.
A new chapter in precision medicine has begun—one where even the cancer cell's own messages can be rewritten for good.
To see article on Phys.org: https://phys.org/news/2026-01-reprogramming-cancer-messenger-era-tumor.html