By fine-tuning the surroundings of single cobalt atoms, researchers reveal how tiny design changes can steer oxygen reactions toward cleaner and more efficient hydrogen peroxide production.
Hydrogen peroxide is an essential chemical used in health care, sanitation, and environmental treatment. Yet, conventional industrial production remains energy-intensive and generates hazardous waste, prompting researchers to seek cleaner and more sustainable alternatives.
A team at National Taiwan University explored an electrochemical approach that converts oxygen into hydrogen peroxide using electricity—a process compatible with renewable energy sources and decentralized production. However, the efficiency and selectivity of this reaction depend heavily on the catalyst design.
The researchers focused on single-atom catalysts, in which isolated cobalt atoms are dispersed across a carbon framework. These catalysts are efficient but highly sensitive to their local atomic environment. When oxygen binds too strongly to the active site, the reaction tends to fully reduce oxygen to water, rather than forming hydrogen peroxide.
The study is published in the Chemical Engineering Journal.
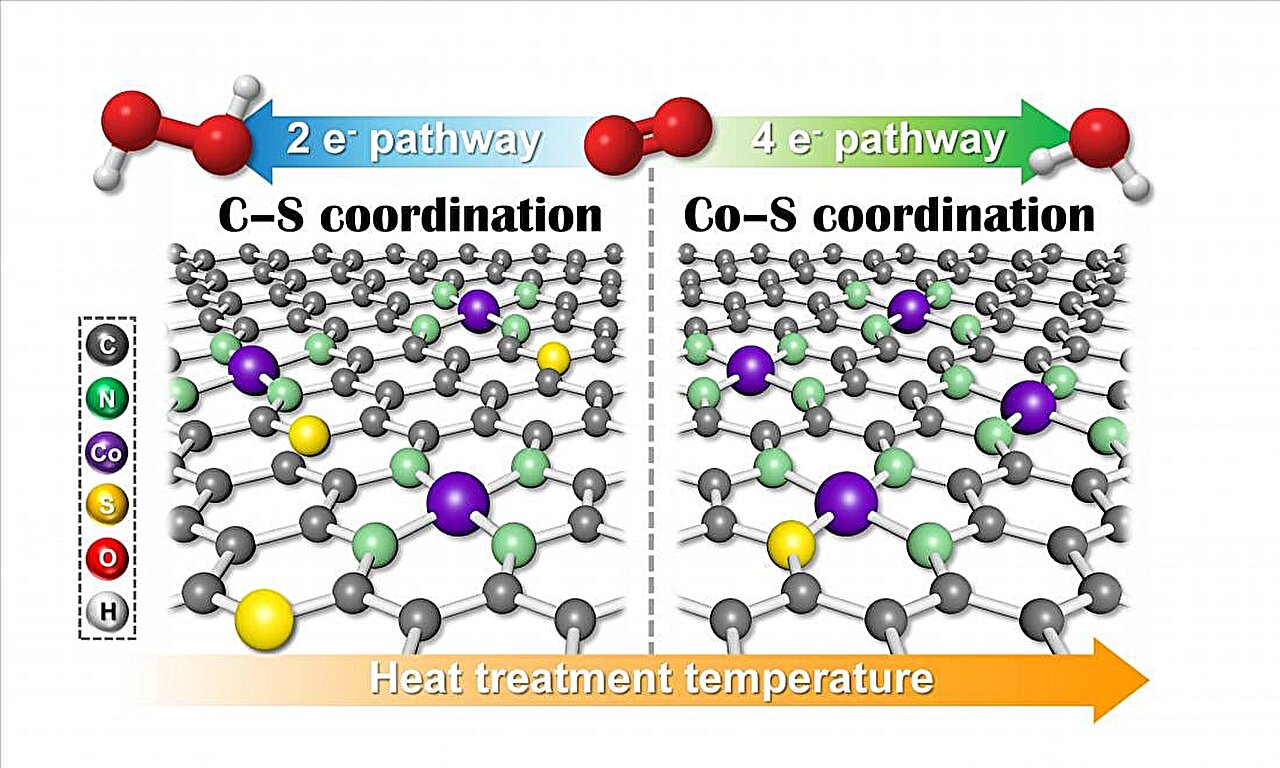
To address this, the team turned to wheat flour as a precursor to engineer the atomic environment around cobalt sites. Gluten proteins in wheat are rich in disulfide bonds—sulfur-based linkages between amino acid chains—that thermally decompose during high-temperature treatment. This process releases sulfur species, which become embedded into the carbon matrix and influence the coordination surroundings of the cobalt sites.
By controlling the pyrolysis temperature, the researchers were able to fine-tune the distance between sulfur atoms and cobalt centers. When sulfur was positioned in the outer coordination shell—close to but not directly bonded with cobalt—the catalyst demonstrated excellent selectivity and structural stability, favoring the two-electron pathway for hydrogen peroxide production.
In contrast, direct cobalt–sulfur bonding led to catalyst deformation and shifted the reaction toward complete reduction of oxygen to water.
"Our goal was to understand how subtle changes in sulfur positioning could reshape the entire catalytic pathway," said Song-Chi Chen, first author of the study. "We found that outer-shell C–S coordination offered the ideal balance—enhancing selectivity without compromising structural stability."
Using advanced X-ray absorption spectroscopy, the team confirmed that the optimized catalyst maintained atomic dispersion throughout operation. The resulting system produced hydrogen peroxide concentrations viable for real-world applications. Its effectiveness was further demonstrated by degrading phenol—an industrial pollutant—through an electro-Fenton process.
"By precisely engineering the coordination environment around single cobalt atoms, we can steer electrochemical reactions toward greener and more targeted outcomes," said co-corresponding author Prof. Chih-Jung Chen, who supervised the research.
To see article on Phys.org: https://phys.org/news/2026-01-atomic-sustainable-hydrogen-peroxide-electrosynthesis.html