Prof. Pi-Tai Chou's group at National Taiwan University Department of Chemistry has created a catalyst that turns two challenges into one solution: it produces clean hydrogen with remarkable efficiency while breaking down urea with ease. This breakthrough not only lowers the energy cost of hydrogen but also helps eliminate harmful pollutants.
Innovative catalyst design and synthesis
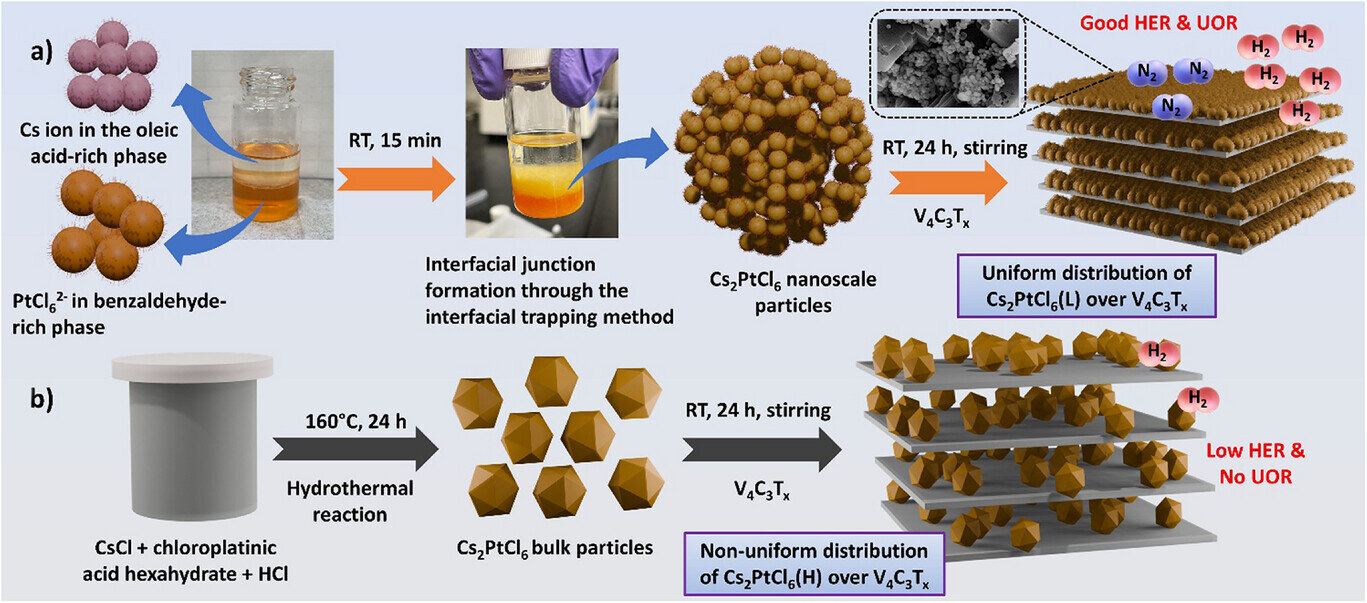
Researchers at National Taiwan University have revealed an innovative method to create a next-generation catalyst by bringing together two powerful materials: V₄C₃Tₓ MXene and tiny Cs₂PtCl₆ perovskite nanoparticles.
Instead of relying on traditional methods, the team used an interfacial trapping strategy that forms the perovskite nanoparticles exactly at the boundary of two liquids, rapidly at room temperature. This gentle but precise approach allows the Cs₂PtCl₆ particles to spread uniformly across the MXene surface, producing a highly connected hybrid structure that would be difficult to achieve otherwise.
The study is published in Angewandte Chemie International Edition.
Exceptional hydrogen production and efficiency
This strong attachment proves to be the key. The engineered Cs₂PtCl₆@V₄C₃Tₓ catalyst shows exceptional performance in producing clean hydrogen, requiring remarkably low energy to start the reaction. Even at small voltages, the material generates hydrogen quickly and consistently, beating many well-known catalysts, including several based on noble metals.
The highly conductive MXene layers help shuttle electrons efficiently, while the perovskite nanoparticles provide active spots where the reaction can happen easily.
Meanwhile, the catalyst demonstrates a powerful ability to break down urea, a compound commonly found in wastewater from agriculture and industry. Instead of acting as a burden, urea becomes an advantage: its oxidation lowers the energy needed for hydrogen production, turning waste into a useful contributor. This dual action means the catalyst can produce clean fuel and help remove pollutants in one integrated process.
"This work shows how smart material design can turn a simple interface into a powerful engine for both clean energy and environmental repair. By coupling hydrogen generation with urea removal, we reveal a strategy that produces value from waste and pushes sustainable technology forward," says Prof. Pi-Tai Chou, the leader of this research team.
To see article on Phys.org: https://phys.org/news/2025-11-interface-driven-catalyst-combines-hydrogen.html